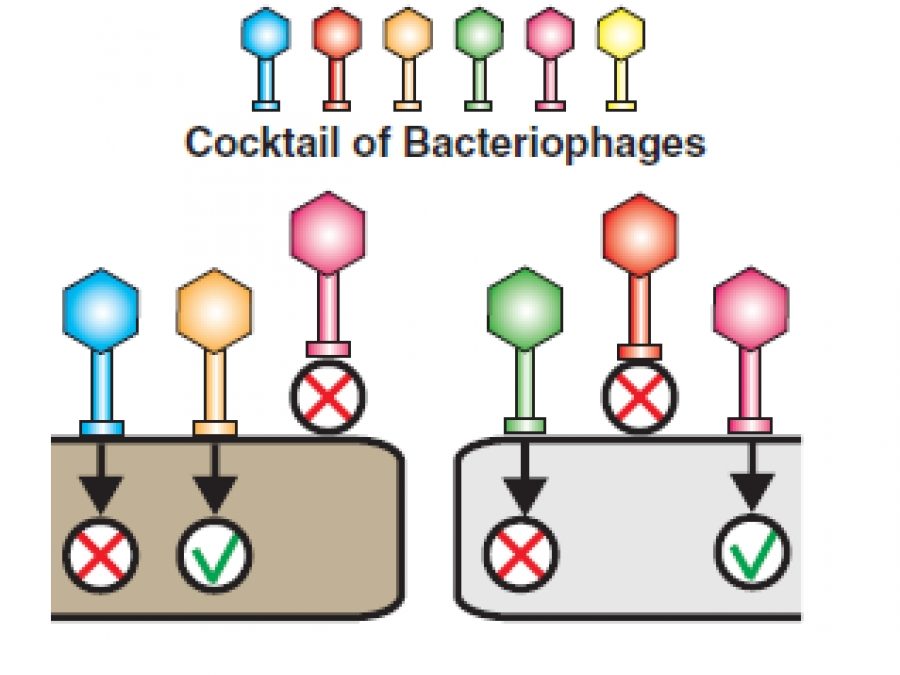
TECHNOPHAGE, a biotechnology company headquartered in Lisbon, Portugal, announced today that the company has received authorization from U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) application for TP-102, a bacteriophage cocktail for the treatment of infected chronic ulcers, namely diabetic foot infections. This clearance will enable TechnoPhage to initiate trials in humans, confirming safety and efficacy of these therapeutics which has already been assessed in laboratory environment. At the moment, no current topical therapy is available specifically targeted at chronic diabetic foot infections. Therefore, TechnoPhage will address an unmet medical need with great impact on the wellbeing of these patients.